Frequency Spectrum In the subject Technology, the topic Frequency Spectrum refers to the range of electromagnetic frequencies used for communication. It is essential for wireless technologies like radio, TV, and mobile networks, ensuring efficient transmission of signals.
Frequency Spectrum
Electromagnetic Waves and Their Properties
- Electromagnetic (e.m.) waves are fundamental to modern communication systems, enabling the transfer of signals through conductors, air, or optical fibers.
- These waves consist of oscillating electric and magnetic fields that propagate through space, governed by Maxwell’s electromagnetic theory.
- Mutual Interaction: A time-varying electric field creates a magnetic field, and vice versa, allowing the wave to propagate.
Mathematical Representation:
- Electric Field: E=E0sin(kz−ωt)
- Magnetic Field: H=H0sin(kz−ωt)
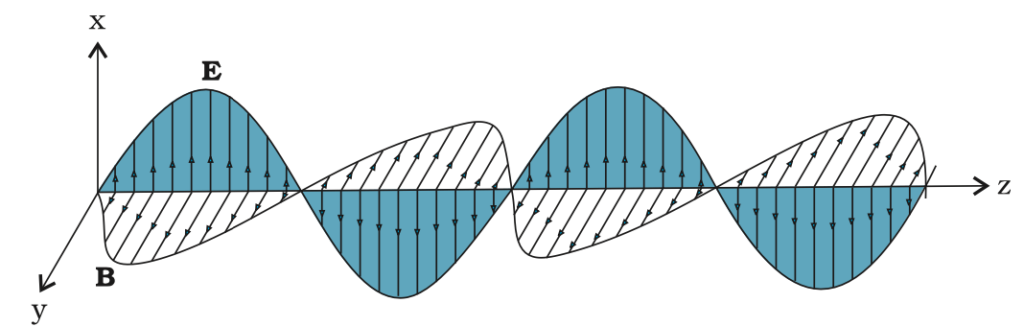
Key Properties of EM Waves:
- Transverse Nature: Electric (E) and magnetic (B) fields oscillate perpendicular to each other and to the direction of wave propagation.
- Do Not Require a Medium: EM waves can travel through a vacuum.
- Example: Light from the Sun reaches Earth.
- Speed in a Vacuum (c): All EM waves travel at a constant speed of approximately 3×108 m/s.
- Relationship: c=λ×f (speed = wavelength × frequency).
- Wavelength and Frequency Relationship: Longer wavelength = Lower frequency = Lower energy.
- Energy and Momentum: EM waves carry energy and momentum, which can be transferred to matter (e.g., solar panels).
- Reflection, Refraction, and Diffraction: EM waves can reflect, refract, or diffract depending on the medium and obstacles.
- Example: Radio waves bend around buildings (diffraction).
- Polarization: The orientation of the electric field can be confined to a specific direction, reducing glare (e.g., polarized sunglasses).
- Interaction with Matter:
- Low Frequency: Penetrates deeply (e.g., radio waves).
- High Frequency: Absorbed or scattered (e.g., UV rays absorbed by the ozone layer).
How e.m. Waves Work in Communication:
- Transmitter: Converts the signal into e.m. waves using an antenna.
- Propagation: Waves travel through space or medium.
- Receiver: Captures the waves using another antenna and extracts the information.
Frequency Spectrum
- The frequency spectrum refers to the range of electromagnetic waves arranged based on their frequencies or wavelengths, used for transmitting signals in communication systems.
- Frequencies are measured in Hertz (Hz): 1 Hz = 1 cycle per second
Regions
The types of electromagnetic radiation are broadly classified into the following classes (regions, bands or types):
- Gamma radiation
- X-ray radiation
- Ultraviolet radiation
- Visible light (light that humans can see)
- Infrared radiation
- Microwave radiation
- Radio waves
- This classification goes in the increasing order of wavelength, which is characteristic of the type of radiation.
- There are no precisely defined boundaries between the bands of the electromagnetic spectrum; rather they fade into each other like the bands in a rainbow.
Divisions of the Frequency Spectrum
| Frequency Band | Frequency Range | Wavelength | Applications |
| Radio Waves | 3 Hz – 300 GHz | >1 mm | AM/FM radio, TV broadcasting, mobile communication. |
| Microwaves | 300 MHz – 300 GHz | 1 mm−1 m | Satellite communication, radar, Wi-Fi, microwave ovens. |
| Infrared (IR) | 300 GHz – 400 THz | 750 nm−1 mm | Remote controls, thermal imaging, fiber optics. |
| Visible Light | 400 THz – 750 THz | 400 nm−750 nm | Optical fiber communication, vision. |
| Ultraviolet (UV) | 750 THz – 30 PHz | 10 nm−400 nm | Sterilization, medical imaging, forensic analysis. |
| X-Rays | 30 PHz – 30 EHz | 0.01 nm−10 nm | Medical imaging, security scans. |
| Gamma Rays | >30 EHz>30EHz | <0.01 nm | Cancer treatment, nuclear research. |
Radio Waves
- Definition:
- Electromagnetic waves with the lowest frequencies and longest wavelengths in the spectrum.
- Frequency: Below 300 GHz
- Wavelength: Greater than 1 mm
- Types:
- Microwaves: Higher frequency (above 1 GHz) and shorter wavelengths (<30 cm).
- Travel Speed:
- In vacuum: Speed of light (c = 3 × 10⁸ m/s)
- In Earth’s atmosphere: Slightly slower due to air properties.
- Production: Created by the accelerated motion of charges in conducting wires
Generation and Reception
- Generation:
- Artificial sources: Radio transmitters connected to antennas.
- Natural sources: Lightning, astronomical objects, and blackbody radiation from warm objects.
- Reception:
- Antenna receives radio waves → Converts them into oscillating electric currents → Sent to the receiver for signal processing.
Why Useful?
- Can pass through atmosphere, buildings, and weather conditions.
- Long wavelengths diffract around obstacles and follow Earth’s surface.
Propagation Characteristics
- Line of Sight: Radio waves travel in a straight line between transmitter and receiver.
- Limited to frequencies above 30 MHz.
- Examples: Cell phones, FM radio, TV broadcasting, radar.
- Visual Horizon: Propagation limited to about 64 km (40 miles) on Earth’s surface.
- Indirect Propagation: Radio waves reach beyond the line of sight by diffraction and reflection.
- Ground Waves: For frequencies below 2 MHz, radio waves can diffract around obstacles (e.g., mountains) and propagate over the horizon.
- Used for mediumwave and longwave broadcasting.
- Very Low Frequency (VLF) and Extremely Low Frequency (ELF) waves can even penetrate water, enabling communication with submerged submarines.
- Skywaves: At medium wave and shortwave, radio waves reflect off the ionosphere and return to Earth.
- Uses: Limited in modern systems but still used by military radar and shortwave radio stations.
Applications of Radio Waves
- Communication: Mobile phones, radios, satellites.
- Navigation: GPS, radar systems.
- Broadcasting: TV, AM/FM radio.
- Scientific Uses: Astronomy, ionospheric studies.
- Medical Applications:
- Diathermy:Deep tissue heating for promoting blood flow.
- Hyperthermia Therapy: Uses radio waves to kill cancer cells.
- Health and Environmental Effects
- Non-ionizing Radiation:
- Primary Effect: Heating of tissues.
- Concerns:
- Prolonged exposure to strong RF fields may cause cataracts or potential thermal damage.
- Shielding :
- Faraday Cage: Blocks radio waves using conductive enclosures.
Frequency Range: 500 kHz to 1000 MHz.
Applications:
- AM Radio: 530 kHz to 1710 kHz.
- Shortwave Bands: Up to 54 MHz.
- FM Radio: 88 MHz to 108 MHz.
- TV Broadcast: 54 MHz to 890 MHz.
- Cellular Phones: Operate in the UHF band.
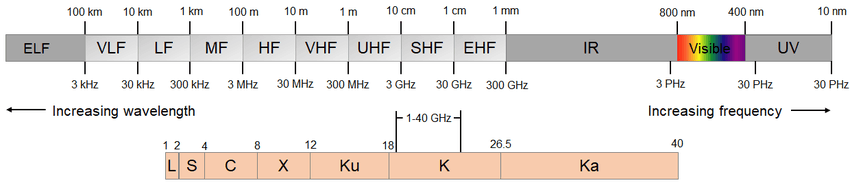
| Band | Frequency Range | Wavelength | Application |
| Extremely Low Frequency (ELF) | < 3 kHz | > 100 km | Electricity |
| Very Low Frequency (VLF) | 3-30 kHz | 100 – 10 km | SONAR |
| Low Frequency (LF) | 30-300 kHz | 10 – 1 km | Marine navigator |
| Medium Frequency (MF) | 300 kHz – 3 MHz | 1 km – 100 m | Medium wave radio |
| High Frequency (HF) | 3-30 MHz | 100 – 1 m | Short wave radio |
| Very High Frequency (VHF) | 30-300 MHz | 10-1 m | FM radio |
| Ultra High Frequency (UHF) | 300 MHz – 3 GHz | 1 m – 10 cm | Commercial TV, cellular mobile, radio |
| Super High Frequency (SHF) | 3-30 GHz | 10-1 cm | Satellite, radar, communication |
Microwave
- Microwaves are a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves.
- Wavelength Range: From 1 meter to 1 millimeter (frequencies between 300 MHz and 300 GHz).
- Common Range: Often defined between 1 and 100 GHz (wavelengths between 30 cm and 3 mm).
- Microwave Spectrum:
- Microwaves are typically divided into frequency bands, including UHF (Ultra High Frequency), SHF (Super High Frequency), and EHF (Extremely High Frequency) bands.
- Common Frequency Bands:
- S, C, X, Ku, K, and Ka bands for radar and satellite communication.
- Produced by devices like klystrons, magnetrons, and Gunn diodes.
Propagation:
- Line-of-Sight: Microwaves travel in straight lines and are limited by the visual horizon (about 40 miles or 64 km on Earth).
- At 100 GHz and above, the atmosphere becomes opaque to microwaves.
Microwave Applications
- Communication: Used in point-to-point communication links (e.g., microwave radio relay networks).
- Satellite Communication:
- Ku-band (12–14 GHz) for television services.
- X-band, Ka-band for military communications.
- Satellite Communication:
- Wireless networks:
- Wi-Fi and Bluetooth operate in the 2.4 GHz ISM band; Wi-Fi 5 (IEEE 802.11ac) uses the 5 GHz band.
- Radar (for air traffic control, weather forecasting, navigation).
- Satellite Navigation:
- Global Navigation Satellite Systems (GNSS), including GPS, GLONASS, and Beidou, use microwave frequencies between 1.2 GHz and 1.6 GHz for broadcasting navigational signals.
- Radio Astronomy:
- Microwaves are used in radio telescopes to study cosmic microwave background radiation (CMBR)from the Big Bang.
- Medical applications (diathermy, cancer treatment).
- Cooking (microwave ovens).
- Industrial heating (drying, curing).
Principle of Microwave Ovens
- Microwave Frequency: Microwaves operate at 3 GHz, resonating with the rotational frequency of water molecules.
- How It Works:
- Energy from microwaves is absorbed by water molecules, raising their kinetic energy and temperature.
- Vibrating water molecules transfer energy to neighboring food molecules, heating the entire food item.
- Advantages Over Conventional Heating:
- Direct heating of food without heating the container.
- More efficient and faster than traditional heating methods.
- Safety Guidelines:
- Porcelain Containers: Safe as they don’t absorb microwaves or conduct electricity.
- Metal Containers: Avoided due to risks of electric shocks or melting.
Health and Safety Considerations
- Non-Ionizing Radiation:
- Main effect: Heating of materials, especially in microwave ovens.
- Biological Effects:
- Eye Sensitivity: The eye’s lens and cornea can absorb microwave energy, potentially leading to cataracts due to heating.
- Other Tissues: High exposure to microwaves can cause burns and damage to deeper tissues due to heating of moisture-rich areas.
Infrared (IR)
- Electromagnetic radiation with wavelengths longer than visible light but shorter than microwaves.
- Wavelength range: 750 nm (400 THz) to 1 mm (300 GHz).
- Divided into:
- Near-IR: 120–400 THz (750–2,500 nm).
- Mid-IR: 30–120 THz (2.5–10 μm).
- Far-IR: 300 GHz–30 THz (10–1 mm).
- Known as heat waves since water, CO₂, and NH₃ absorb them, increasing thermal motion.
- Plays a role in the greenhouse effect:
- Earth absorbs visible light and re-radiates it as infrared.
- Infrared radiation is trapped by greenhouse gases, maintaining Earth’s temperature.
- Invisible to the human eye.
- IR radiation is emitted by objects through Rotational-vibrational molecular movements.
Applications of Infrared Radiation
- Night Vision: Enhanced visibility in low light.
- Active IR Illumination: Uses infrared light sources to enhance visibility.
- Example: Used in military, firefighting, and security.
- Thermography (Thermal Imaging):
- Detects temperature differences based on black-body radiation.
- Applications:
- Military: Surveillance and target tracking.
- Industrial: Detecting heat loss, overheating equipment.
- Medical: Blood flow monitoring, firefighting.
- Hyperspectral Imaging:
- Captures full IR spectra at each pixel.
- Used in geological studies, biological analysis, and UAV surveillance.
- Heating:
- Infrared heaters used in industrial processes: Plastic welding, curing, annealing, and print drying.
- Infrared saunas and aircraft de-icing.
- Cooling:
- Passive Daytime Radiative Cooling (PDRC):
- Reflects sunlight, radiates heat to space.
- Can mitigate global warming.
- Passive Daytime Radiative Cooling (PDRC):
- Communications:
- Remote Controls: Emitted by LEDs in TV remotes, video recorders, etc.
- Data Transmission: IrDA standards for short-range communication.
- Optical Fiber: Infrared lasers transmit data efficiently (~1,330–1,550 nm).
- Spectroscopy:
- Identifies molecular vibrations and chemical bonds.
- Common in organic compound analysis and material characterization.
Infrared in Biology and Medicine
- Biological Systems:
- IR sensing in snakes, vampire bats, and jewel beetles for prey detection.
- Some fish use near-IR for navigation and hunting.
- Photobiomodulation: Near-IR light aids in:
- Wound healing.
- Chemotherapy-induced ulcer treatment.
- Potential nervous system healing.
- Health Hazards:
- High IR exposure can cause eye damage or blindness.
- Protective goggles required in high-heat industrial settings.
Infrared in Technology and Science
- Astronomy:
- IR telescopes detect Protostars, planetary heat, and distant galaxies.
- Study of cosmic microwave background radiation for Big Bang insights.
- Meteorology:
- Weather satellites use IR to:
- Determine cloud height/type, surface temperatures.
- Track El Niño and ocean currents.
- Weather satellites use IR to:
- Art Conservation:
- Infrared reflectography reveals:
- Underdrawings in paintings.
- Hidden details in historical manuscripts.
- Infrared reflectography reveals:
- Cleaning:
- Infrared cleaning in scanners removes dust and scratches.
Visible Light
- The portion of the electromagnetic spectrum (EM) detectable by the human eye.
- Wavelength range: 380 nm to 760 nm.
- Colors: Violet → Blue → Green → Yellow → Orange → Red.
- Frequency range: 400–790 THz.
Key Characteristics of Visible Light
- Peak Emission by the Sun:
- The Sun emits its peak power in the visible spectrum, though it emits slightly more energy in infrared overall.
- Colors of the Spectrum:
- Visible light consists of colors ranging from violet (shortest wavelength) to red (longest wavelength).
- Rainbows: Visible light forms a rainbow, with infrared just beyond red and ultraviolet beyond violet.
- White Light: A combination of all wavelengths in the visible spectrum.
- Prism Effect: Passing white light through a prism splits it into its constituent colors.
- Biological Importance:
- Vision: Visible light excites electrons in molecules and atoms, triggering energy-level transitions. These transitions underlie the mechanism of human vision.
- Vision: Light reflects → Eye detects → Brain interprets (shades, hues).
- Photosynthesis: Absorption of specific wavelengths powers chemical reactions in plants.
- Vision: Visible light excites electrons in molecules and atoms, triggering energy-level transitions. These transitions underlie the mechanism of human vision.
Applications of Visible Light
Everyday Uses
- Illumination:
- Natural light from the Sun.
- Artificial lighting (e.g., bulbs, LEDs) for homes, offices, and streets.
- Vision:
- Enables humans and animals to see and interact with their surroundings.
Communication
- Optical Fiber Technology:
- Transmits information (e.g., internet data) using light pulses, often in the near-infrared range.
- Offers high-speed communication with minimal signal loss.
- Signal Lights:
- Traffic lights, lighthouses, and communication signals use visible light to convey messages.
Imaging and Entertainment
- Photography and Videography:
- Cameras capture images by detecting visible light.
- Projectors and screens display images and videos by emitting visible light.
- Holography:
- Uses visible light for creating 3D holograms in art, security, and data storage.
Endoscopy:
- Uses visible light to visualize internal organs through a thin, flexible tube.
Science and Research
- Spectroscopy:Analyzes the interaction of visible light with matter to study chemical composition.
- Astronomy: Optical Telescopes observe celestial bodies in the visible spectrum to study stars, planets, and galaxies.
- Microscopy: Light microscopes use visible light to magnify objects for biological and material studies.
Agriculture
- Greenhouse Lighting: Visible light (or artificial grow lights) supports photosynthesis in plants.
Color Printing and Displays:
- Printers and digital screens use the visible spectrum to reproduce colors.
Industrial Applications
- Laser Cutting and Welding: Uses high-intensity visible light for precise cutting and joining of materials.
Safety and Security
- Visible Laser Pointers: Used for presentations and as guiding tools in various operations.
- Surveillance Cameras: Detect visible light to monitor areas for security.
Education and Entertainment
- Displays: Screens, projectors, and VR systems emit visible light for educational content and immersive experiences.
- Laser Shows: Entertainment events use visible light lasers to create vibrant visual effects.
Ultraviolet (UV) Radiation
- Wavelength Range: 10–400 nanometers.
- Frequency Range: Higher than visible light, lower than X-rays.
- Energy: UV photons carry energy between 3.1 to 12 electron volts, sufficient for ionizing or inducing chemical reactions.
Types of UV Radiation
- UVA (315–400 nm):
- Least harmful; reaches Earth’s surface.
- Causes tanning and contributes to skin aging.
- UVB (280–315 nm):
- Partially absorbed by the ozone layer.
- Causes sunburn and DNA damage.
- UVC (100–280 nm):
- Completely absorbed by Earth’s atmosphere.
- Highly ionizing, used for sterilization.
History and Discovery
- Discovered by Johann Wilhelm Ritter in 1801.
- First termed as “chemical rays” due to their reactivity.
Sources:
- Natural:The Sun Emits UV radiation constituting ~10% of its total energy.
- Atmosphere’s Role:
- Blocks UVC completely and most UVB.
- UVA primarily reaches the surface (~95% of UV radiation reaching Earth).
- Atmosphere’s Role:
- Artificial: Mercury-vapor lamps, black lights, tanning lamps, and Cherenkov radiation.
Applications of Ultraviolet Radiation
- Health
- Vitamin D Synthesis: UVB triggers vitamin D synthesis in the skin.
- Recommended: 5–15 minutes of sunlight exposure 2–3 times a week.
- Moderate exposure aids bone health and mood regulation.
- Phototherapy: treats skin conditions like psoriasis, eczema, and vitiligo.
- Precision: UV is used in LASIK eye surgery for focused beams.
- Vitamin D Synthesis: UVB triggers vitamin D synthesis in the skin.
- Disinfection and Sterilization
- Germicidal Lamps: UVC effectively sterilizes surfaces, air, and water.
- Healthcare: Pulsed UV light or far-UVC (222 nm) for pathogen reduction, including SARS-CoV-2.
- Forensics:
- Detection of bodily fluids (blood, saliva).
- Authentication of documents and counterfeit currency via UV-sensitive markers.
- Astronomy:
- UV telescopes study hot stars and distant galaxies.
- Animal Vision:
- Birds, bees, and reptiles detect near-UV for communication and survival.
- Lithography:
- Used in semiconductor manufacturing (Microchips) and circuit boards.
- Analytical Chemistry:
- UV spectroscopy for structural analysis (proteins, nucleic acids).
- Detection of environmental pollutants in water and air.
- Air Purification:
- UV oxidation breaks down VOCs and pollutants.
- Polymer Processing:
- UV curing hardens adhesives and coatings quickly.
- Used in dental fillings, inks, and optical fiber coatings.
- UV Photography: Captures faded ink and hidden documents via fluorescence.
- Fluorescent Dyes: Used in textiles, papers, and forensic sprays (e.g., pepper spray).
- Fluorescent Art: Blacklight paints and UV effects in amusement parks.
- Fire Detection: UV sensors detect “solar-blind” wavelengths in fires.
Harmful Effects:
- Skin Damage:
- Sunburn and Tanning: UVB causes DNA damage and increases skin cancer risks.
- Aging: UVA breaks down collagen, accelerating aging.
- Eye Damage:
- UVC and UVB can cause photokeratitis (e.g., snow blindness) and contribute to cataracts.
- Immune Suppression:
- UVA suppresses immune responses and increases risks of skin diseases.
- Degradation of Materials:
- Polymers & Dyes: UV causes fading, cracking, and loss of strength in materials like ropes and plastics.
- Deteriorates polymers, pigments, and dyes.
- Museums and conservationists use UV-blocking materials to preserve artifacts.
UV Blocking Mechanisms
- Ozone Layer: Absorbs all UVC and most UVB radiation.
- Ozone Depletion: Chlorofluorocarbons (CFCs) damage the ozone layer, increasing UV exposure, which is a global issue.
- Materials:
- Sunscreens (e.g., zinc oxide, titanium dioxide).
- Protective glass blocks UV below 300 nm.
- Clothing with UPF ratings reduces UV exposure.
X-rays
- Definition: High-energy electromagnetic radiation with wavelengths shorter than UV and longer than gamma rays (10 nm to 10 pm).
- Discovered by Wilhelm Conrad Röntgen in 1895.
- Properties:
- Ionizing radiation (can damage DNA).
- Can penetrate solids like construction materials and living tissue.
- Used in medical diagnostics and material science.
- Associated with risks like cancer and burns at high exposure.
Medical Uses of X-rays
- Projectional Radiography:
- Bone Imaging: Detect fractures, pneumonia, lung diseases, and more.
- Dental Radiography: Detect cavities and other oral issues.
- Abdominal X-rays: Detect intestinal obstructions or free fluid.
- Computed Tomography/CT Scans :
- Cross-sectional 3D images of internal body structures.
- Fluoroscopy:
- Real-time imaging used in procedures like cardiac catheterization and barium swallow for swallowing disorders.
- Radiotherapy:
- High-dose X-rays treat cancers, targeting malignant cells with higher radiation doses than imaging.
Other Uses of X-rays
- X-ray Crystallography:
- Used to determine the atomic structure of materials and molecules (e.g., DNA structure discovery).
- X-ray Astronomy:
- Studies celestial objects emitting X-rays (e.g., stars, black holes).
- Industrial & Quality Control:
- Industrial Radiography: Inspects materials (e.g., welds, pipes) for defects or weak spots.
- CT in Industry: Creates 3D models for internal inspection.
- Security:
- Airport baggage scanners use X-rays for security screening of luggage.
- Border control scanners inspect truck interiors
- Fine Art & Cultural Preservation:
- X-rays are used to examine paintings and other artifacts for restorations or hidden layers beneath the surface.
- Authentication:
- Quality control in manufacturing and detecting counterfeit products (e.g., chips, currency, art) by inspecting internal structures.
Advantages and Limitations
- Advantages:
- Non-invasive and Quick: X-ray imaging is fast, making it ideal for emergency situations.
- High Resolution: Can capture fine details, especially in dense materials like bone.
- Limitations:
- Radiation Exposure: X-rays involve ionizing radiation, which can cause tissue damage and increase the risk of cancer if used excessively.
- Cost and Accessibility: Advanced X-ray techniques like CT scans may be expensive and not available everywhere.
Adverse Effects
- Cancer Risk:
- X-rays are classified as carcinogens.
- High radiation doses from CT scans increase cancer risk.
- Fetal Risks:
- X-rays can harm a fetus, so alternatives (like ultrasound) are used during pregnancy.
- Radiation Exposure:
- Regular exposure from diagnostic X-rays can contribute to radiation-induced cancers.
- Protective Measures:
- Use of lead shielding, limiting exposure, and avoiding unnecessary scans.
Gamma Rays
- Definition: High-energy electromagnetic radiation with wavelengths shorter than X-rays.
- Wavelength: Less than 10 picometers
- Frequency: Above 30 exahertz (3×10^19 Hz)
- Photon Energy: 100 keV to 8 MeV
- Penetration: Highly penetrating, requiring dense materials like lead or concrete for shielding.
Discovery of Gamma Rays
- 1900: Gamma rays were discovered by French scientist Paul Villard while studying radium.
- 1903: Ernest Rutherford named the radiation gamma rays, distinguishing them from the previously discovered alpha and beta rays.
Properties
- Ionizing Radiation:
- Gamma rays can cause DNA mutations, cancer, and organ damage.
- High doses lead to burns and radiation sickness.
- Penetration:
- Gamma rays pass through the body, posing a challenge for radiation protection.
- Interaction with Matter:
- Photoelectric effect: Gamma photon ejects an electron from an atom.
- Compton scattering: Gamma photon scatters after transferring energy to an electron.
- Pair production: At energies above 1.02 MeV, gamma photons produce electron-positron pairs.
Sources
- Natural Sources:
- Radioactive decay: Naturally occurring radioisotopes like potassium-40, cobalt-60 decay.
- Terrestrial gamma-ray flashes from thunderstorms.
- Cosmic sources: Gamma rays from cosmic rays, pulsars, and supernovae.
- Artificial Sources:
- Nuclear reactors (fission)
- High-energy physics experiments : Large Hadron Collider produce gamma rays from particle interactions.
- In Particle Physics
- Electron-Positron Annihilation: When an electron and a positron annihilate each other, gamma rays are emitted.
Applications
- Medical Uses:
- Gamma-knife surgery: Used in treating brain tumors by directing concentrated gamma rays at the tumor, minimizing damage to surrounding tissues.
- Diagnostic Imaging: Used in Positron Emission Tomography (PET) scans, where gamma rays emitted by radiolabeled substances are used to image the body.
- The most common gamma emitter used in medical applications is Technetium-99m.
- Sterilization: Gamma rays are used to sterilize medical equipment and food.
- Cancer Treatment: Gamma rays are used to destroy cancer cells in radiation therapy.
- Industrial Uses:
- Non-destructive testing: Gamma rays inspect materials, detect flaws in parts, and measure densities (e.g., in mining, chemicals).
- Food irradiation: Gamma radiation is used to kill bacteria and prevent food spoilage.
- Astronomy:
- Gamma-ray astronomy: Observing celestial phenomena like gamma-ray bursts and supernovae.
- Security:
- Container security: Gamma-ray scanners are used for detecting hidden items in shipping containers.
Health Effects
- Low-Dose Exposure:
- Increased risk of cancer and genetic mutations. Example: Nuclear workers may experience a slight increase in cancer risk.
- High-Dose Exposure:
- Guaranteed damage (e.g., radiation sickness, hair loss).
Shielding and Protection
- Lead: Due to its high density and atomic number, lead is commonly used to shield against gamma radiation.
- Depleted Uranium: In some cases, it is used for shielding due to its superior stopping power for gamma rays.
Uses of Specific Frequency Bands in Communication
- Radio Broadcasting:
- AM Radio: Medium frequency (300 kHz – 3 MHz).
- FM Radio: Very high frequency (30 MHz – 300 MHz).
- Television Broadcasting:
- VHF and UHF bands (30 MHz – 3 GHz).
- Mobile Communication:
- UHF band (300 MHz – 3 GHz) for 2G, 3G, 4G.
- SHF band (3 GHz – 30 GHz) for 5G.
- Satellite Communication:
- SHF (3 GHz – 30 GHz) and EHF (30 GHz – 300 GHz).
- Navigation:
- VLF and LF for aviation and maritime navigation.
- UHF for GPS systems.
- Military Communication:
- HF for long-range communication.
- SHF for radar systems.
Spectrum Allocation and Management
- What is Spectrum Allocation?
- The process of assigning specific frequency bands to different types of communication services to avoid interference.
- Managed by international and national regulatory bodies.
- Regulatory Bodies:
- International Telecommunication Union (ITU): Oversees global spectrum allocation.
- India: The Wireless Planning and Coordination Wing (WPC) of the Ministry of Communications is the National Radio Regulatory Authority responsible for frequency spectrum management, including licensing and caters the needs of all wireless users.
- Example of Spectrum Allocation in India:
- Mobile operators bid for frequency bands during spectrum auctions for 4G/5G services.
- Spectrum pricing depends on demand, frequency range, and technology.
