Depletion of the Ozone Layer is an important topic in World Geography, focusing on the thinning of the ozone layer that protects the Earth from harmful Ultraviolet (UV) rays. This topic covers the causes of ozone layer depletion, the ozone depleting potential and global warming potential of various ozone-depleting substances, and their long-term environmental impacts. Understanding the effects of ozone layer depletion is essential for grasping global environmental challenges and climate protection efforts.
Depletion of the ozone layer
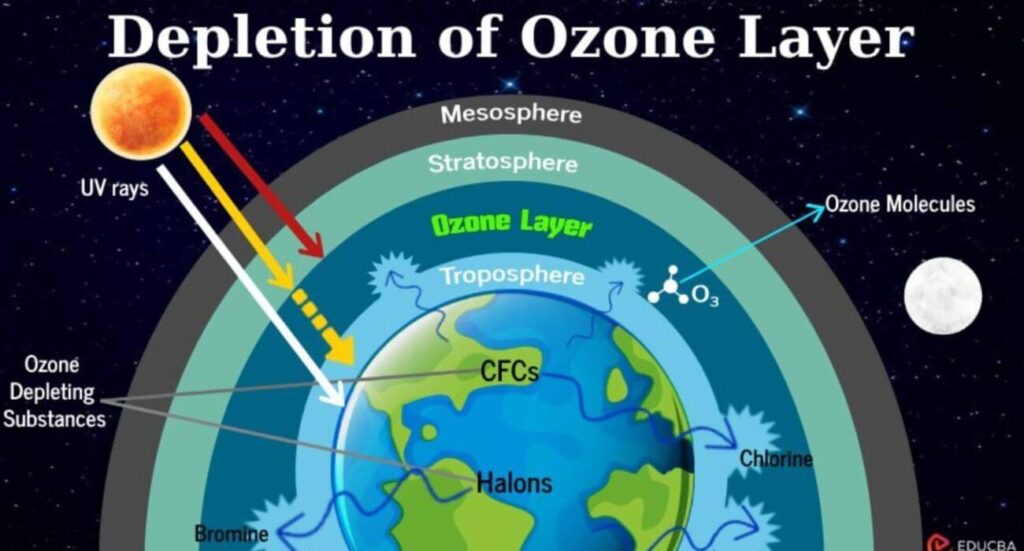
Ozone layer
- The ozone layer is a thin layer in the Earth’s atmosphere, located in the stratosphere (15 to 35 km above the Earth’s surface). It contains a high concentration of ozone (O₃) gas and plays a crucial role in absorbing harmful ultraviolet (UV-B and UV-C) radiation from the Sun, thereby protecting life on Earth.
- The ozone molecules continuously break down and reform due to ultraviolet radiation, a chemical process that gives the stratosphere the name “Chemosphere“.
Ultraviolet (UV) Rays
- UV rays are a type of electromagnetic radiation emitted by the Sun.
- Their wavelength ranges from 100 to 400 nm.
- UV Rays are of 3 Types
- UV-A: 315-400 nm
- UV-B: 200-315 nm
- UV-C: 100-280 nm – The most harmful rays but absorbed by the ozone layer.
- Stratospheric ozone is called “Good Ozone” because it absorbs 95% of harmful UV rays, protecting life on Earth.
- Hence, it is also known as “Earth’s Protective Shield” or “Earth’s Umbrella.”
Ozone Formation:
- Ozone (O₃) is formed when oxygen molecules (O₂) absorb UV radiation and break into oxygen atoms (O), which then combine with O₂ to form ozone (O₃).
O₂ + UV → 2O
O + O₂ → O₃
Causes of Ozone Layer Depletion
- Halogenated gases are the primary culprits behind ozone depletion. These include: Chlorofluorocarbons, chlorine, bromine, methyl chloroform, carbon tetrachloride etc.
- One chlorine atom can destroy 100,000 ozone molecules.
- Chlorine atoms are released from CFCs due to UV radiation in the stratosphere.
- Increased CFCs and Halons accelerate ozone depletion.
- Chlorine and bromine atoms break ozone molecules through multiple chemical reactions.
- This leads to the thinning of the ozone layer, known as the Ozone Hole.
- CFCl₃ is affected by the sun’s ultraviolet rays (UV rays).
- The ultraviolet rays break the bonds present in the CFCl₃ molecule.
- As a result, a chlorine free radical (Cl•) and a CFCl₂ molecule are produced from CFCl₃.
CFCl₃+hv→CFCl₂+Cl•
- The simplest example of such a cycle involves a chlorine atom reacting with an ozone molecule, taking one of its oxygen atoms to form ClO and releasing an oxygen molecule. Chlorine monoxide (ClO) reacts with another ozone molecule (O3) to form another chlorine atom and two oxygen molecules.
Cl•+O₃→ClO•+O₂
ClO•+O₃→Cl•+2O₂
Unit and Measurement of Ozone
- Atmospheric ozone is measured using a Dobson Spectrophotometer and is expressed in Dobson Units (DU).
- One Dobson Unit (DU) equals a 0.01 mm thick layer of pure ozone at standard temperature and pressure.
Atmospheric ozone is measured by –
- Dobson Spectrometer
- Filter Ozone Meter
- Ozone Mapping Spectrometer
Ozone hole
- The ozone hole is not a literal hole with no ozone but rather a region of significant ozone depletion in the stratosphere over Antarctica during the Southern Hemisphere’s spring (August-October).
- The average ozone concentration in the atmosphere is about 300 Dobson Units (DU).
- Any region where ozone concentration drops below 220 DU is considered part of the ozone hole.
- The ozone hole over Antarctica was first identified in 1985 by the British Antarctic Survey team led by Joseph Farman.
The reason for maximum ozone depletion over Antarctica
- Polar Vortex Effect: A large cyclone-like air circulation pattern around the poles. The temperature in the polar vortex drops to -80°C, leading to the formation of Polar Stratospheric Clouds (PSCs), which store ozone-depleting chlorine compounds.
- Polar Stratospheric Clouds (PSCs): are also called mother of pearl clouds” form in polar stratosphere. They provide surfaces for chemical reactions involving chlorine compounds and contain nitric acid and sulfuric acid. These processes accelerate ozone depletion by activating chlorine and indirectly slowing ozone formation. More common in the Southern Hemisphere due to a stronger polar vortex.
- Effect of Active Chlorine: Due to atmospheric circulation, ozone-depleting gases accumulate in the cold polar vortex. These cold conditions trigger chemical reactions, releasing active chlorine, which accelerates ozone depletion.
List of Ozone Depleting Substances
- CFCs (Chlorofluorocarbons)
- HCFCs (Hydrochlorofluorocarbons)
- Halon
- HBFCs (Hydrobromofluorocarbons)
- CCl4 (Carbon Tetrachloride)
- CH3CCl3 (Methyl Chloroform)
- CH3Br (Methyl Bromide)
- CH2BrCl (Bromochloromethane)
- NOx (Nitrogen Oxide)
Ozone depleting potential and global warming potential of various ozone depleting substances
| Substance | Experiment/use | Ozone Depleting Potential (DU) | global warming potential |
| Chlorofluorocarbons(CFC) | Used as a bleaching agent in the manufacturing of refrigerants, cleaning solvents, aerosol propellants and plastic foams | 6-10 | 4,680-10,720 |
| Halons | Used in fire fighting and explosive safety systems | 3-10 | 1,620-7,030 |
| Carbon tetrachloride(CCl4) | Used in production of CFCs, solvents, fire extinguishers | 1.1 | 1,380 |
| Methyl chloroform(CH3CCI3) | Used as an industrial solvent for cleaning, in inks | 0.1 | 144 |
| methyl bromide(CH3Br) | Use in pesticides and crop disease prevention | 0.6 | 5 |
| Hydrochlorofluorocarbons (HCFC) | Used in refrigerants as substitute for CFC as solvents, in plastic foam manufacturing, as bleaching agent | 0.001-0.5 | 76-2,270 |
| Hydrofluorocarbons (HFC) | Used as CFC replacement in refrigerants, aerosol propellants, fire extinguishers | 0 | 122-14,130 |
Effects of Ozone Layer Depletion
- Increased Exposure to UV Radiation: Leads to skin diseases, cancer, sunburn, cataracts, premature aging, and weakened immunity.
- Impact on Marine Ecosystems: UV radiation damages phytoplankton, affecting the entire marine food chain.
- Negative Effect on Photosynthesis: Reduces plant growth and agricultural productivity.
- Decline in Crop Yield and Quality: Certain crops are highly sensitive to UV exposure, leading to lower production.
- Weakened Immune System: Increased UV exposure reduces disease resistance in humans and animals.
- Temperature Increase in Equatorial Regions: Causes extreme heat, which affects physical and mental development.
Ozone chronology
- 1930: Chapman Cycle discovery (explaining ozone formation & depletion).
- 1970: British Antarctic Survey predicts ozone layer depletion.
- 1974: Role of CFCs in ozone depletion identified.
- 1985: Joseph Farman’s team provides direct evidence of ozone depletion over Antarctica.
Efforts to save the ozone layer
Vienna Convention
- Came into effect in 1988.
- Objective: Protection of the ozone layer and reduction in CFC use.
- Non-binding agreement.
- First international treaty with a universal verification system.
Toronto World Conference – (1988) – Canada
- Called for a 20% reduction in CFC usage by 2005 to tackle global warming and ozone depletion.
Helsinki Conference-1989
- Held on May 2, 1989, to accelerate efforts to reduce CFC use.
- March 2, 1990: A seminar on “Ozone Layer Protection” was held in London.
Montreal Agreement –
- Adopted on September 16, 1987 (celebrated as World Ozone Day).
- Came into effect in 1989.
- India signed the protocol in 1992.
- Binding agreement on reducing ODS(Ozone Depleting Substances) emissions.
- 197 countries signed the protocol.
- UNEP played a key role in implementation.
- Developed countries were required to completely phase out CFC production & usage by 2000.
- Developing countries were given time until 2010 to stop CFC production & consumption.
- Since 2010, the focus has shifted to phasing out HCFCs (Hydrochlorofluorocarbons).
Kigali Agreement 2016
- Objective: Reduce emissions of HFCs (Hydrofluorocarbons), which contribute to global warming.
- Target: Reduce global warming by 0.5°C by 2100.
- Legally binding from 2019.
- All 197 signatory countries of the Montreal Protocol agreed to phase out HFCs by 2047.
- Developed countries began reducing HFCs from 2019, China from 2024, and India from 2028.
- Goals for India
- August (2021) India’s decision to ratify the Kigali Agreement on the phase-out of hydrofluorocarbons (HFCs).
- India must stabilize the use of HFCs from 2028 and reduce it by 80% from 2025 levels by 2047.
- India’s timetable for reducing HFCs use is as follows –
- 2028: Stabilise the use of HFCs.
- 2032: 10% reduction from 2025 levels.
- 2037: 20% reduction from 2025 levels.
- 2042: 30% reduction from 2025 levels.
- 2047: 80% reduction from 2025 levels.
Note-
- Carbon Tetrachloride (CCl₄), a harmful ozone-depleting chemical, is used by steel manufacturing units.
- UNDP is assisting India in phasing out HCFCs by 2030, showing India’s commitment to the Montreal Protocol.
