Nuclear Fission and Fusion are important concepts in Physics that explain how energy can be released from the nucleus of an atom. Nuclear fission is the splitting of a heavy nucleus into lighter nuclei, while nuclear fusion is the combining of light nuclei to form a heavier one. Both processes are vital in understanding nuclear energy and its applications in power generation and stars.
Previous year Questions
| Year | Question | Marks |
| 2021 | Differentiate between nuclear fission and fusion. | 2M |
| 2018 | Write the basic nuclear processes underlying (β−) and (β+) decay. | 2M |
Atomic Structure
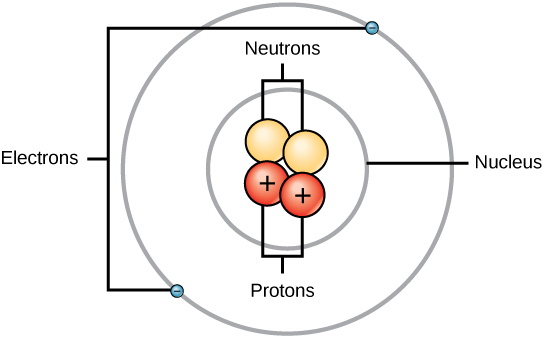
- Nucleus: The nucleus contains most of the atom’s mass but occupies a much smaller space compared to the atom itself.
- The radius of the nucleus is approximately 10⁴ times smaller than the atomic radius.
- The nucleus contains more than 99.9% of the atom’s mass.
Atomic Mass and Units:
- The mass of an atom is very small, e.g., the mass of a carbon atom (¹²C) is 1.992647 × 10⁻²⁶ kg.
- The atomic mass unit (u) is used to express atomic masses:
- 1 u = 1/12th of the mass of a ¹²C atom
- 1 u = 1.660539 × 10⁻²⁷ kg
Neutron Discovery:
- The neutron was discovered in 1932 by James Chadwick:
- Mass of neutron = 1.00866 u = 1.6749 × 10⁻²⁷ kg
- Neutrons are neutral particles with mass similar to protons.
- Free neutrons are unstable and decay into protons, electrons, and antineutrinos with a mean lifetime of about 1000 seconds.
Nuclear Composition:
- The composition of the nucleus involves protons and neutrons:
- Z = Atomic number = number of protons
- N = Neutron number = number of neutrons
- A = Mass number = Z + N (total number of protons + neutrons)
- Notation for nuclides:
- Nuclides are represented as ZXA where X is the chemical symbol, Z is the atomic number, and A is the mass number.
- Example: The nucleus of gold (Au) is represented as ¹⁹⁷₇₉Au (79 protons and 118 neutrons).
Isotopes and Isobars:
- Isotopes: Same number of protons, different number of neutrons. Example:
- Hydrogen (H) has three isotopes:
- Protium (¹H): 1 proton, 1.0078 u (most abundant, 99.985%).
- Deuterium (²H): 1 proton, 1 neutron
- Tritium (³H): 1 proton, 2 neutrons
- Chlorine has two isotopes with mass numbers 34.98 u (75.4% abundant) and 36.98 u (24.6% abundant).
- The average atomic mass of chlorine is calculated as: (75.4 × 34.98 + 24.6 × 36.98) / 100 = 35.47 u
- Hydrogen (H) has three isotopes:
- Isobars: Same mass number, different atomic numbers.
- Example: ¹³₆C and ¹³₇N are isobars (both have a mass number of 13, but different atomic numbers).
- Isotones: Same number of neutrons, different atomic numbers.
- Example: ¹⁹⁸₈₀Hg and ¹⁹⁷₇₉Au are isotones (both have 118 neutrons but different atomic numbers).
SIZE OF THE NUCLEUS
- Rutherford postulated the existence of the atomic nucleus based on his gold foil experiment.
- Geiger and Marsden performed scattering experiments using α-particles:
- The experiment revealed that the distance of closest approach to a gold nucleus by a 5.5 MeV α-particle is about 4.0 × 10⁻¹⁴ m.
- Conclusion: The size of the nucleus must be smaller than 4.0 × 10⁻¹⁴ m.
- Rutherford reasoned that the nucleus was responsible for the scattering of α-particles due to Coulombic repulsion between the positively charged α-particle and the positive charge in the nucleus.
- Effect of Higher Energy α-Particles:
- If we use α-particles with energies higher than 5.5 MeV, the closest approach to the nucleus would decrease.
- As the energy increases, the scattering pattern will start to differ from Rutherford’s predictions, signaling the influence of short-range nuclear forces, in addition to Coulomb repulsion.
- Electron Scattering Experiments: Scattering experiments using fast electrons instead of α-particles can help measure the size of nuclei of different elements accurately.
- Conclusion from experiments:
- A nucleus of mass number A has a radius given by the relation:
- R=R0A1/3
- R0=1.2×10-15 m, where R0 is the constant proportionality factor.
- Implication: The volume of the nucleus is proportional to A, meaning nuclei are roughly spherical and their sizes increase with mass number A.
- Conclusion from experiments:
- Density of Nucleus: Since the volume of the nucleus is proportional to A, and the density remains constant across all nuclei, this means:
- The nuclear matter density is approximately: 2.3×1017 kg/m3
MASS-ENERGY AND NUCLEAR BINDING ENERGY
Mass-Energy Equivalence
- Einstein’s Theory of Special Relativity:
- In his theory of special relativity, Einstein demonstrated that mass is a form of energy.
- Mass-energy equivalence is expressed by the famous equation:
E=mc2
- Where:
- E is the energy equivalent of mass m,
- c is the speed of light in a vacuum (3×108 m/s).
- This relationship shows that mass can be converted into energy, and energy can be converted into mass.
Nuclear Binding Energy
- Nuclear Mass vs. Sum of Proton and Neutron Mass:
- The mass of a nucleus is always less than the sum of the masses of its individual protons and neutrons.
- For instance, consider the nucleus of Oxygen-16 (8O16) which has 8 protons and 8 neutrons:
- Mass of 8 protons: 8×1.00727 u=8.05776u
- Mass of 8 neutrons: 8×1.00866 u=8.06928u
- Mass of electrons: 8×0.00055 u=0.0044u
- Expected mass of O16 nucleus (without binding): 16.12744 u
- Experimental mass from spectroscopy: 15.99493 u
- Mass defect: 16.12744 u−15.99053 u=0.13691 u
- Mass Defect and Binding Energy:
- The mass defect (ΔM) is the difference between the total mass of the individual nucleons and the mass of the nucleus.
- This difference represents missing mass, which has been converted into binding energy.
ΔM=[Z⋅mp+(A−Z)⋅mn]−M
where:
- Z = number of protons
- A = mass number
- mp = mass of a proton
- mn = mass of a neutron
- M = mass of the nucleus.
Binding Energy (Eb) is the energy required to separate a nucleus into its nucleons.
Eb=ΔM⋅c2
- Example: In the case of oxygen-16, the mass defect ΔM results in the release of binding energy when protons and neutrons bind together.
Binding Energy per Nucleon
- It represents the average energy required to remove a nucleon from the nucleus.
Ebn=Eb/A
- Where:
- Ebn is the binding energy per nucleon,
- A is the mass number (total number of nucleons).
Key Observations:
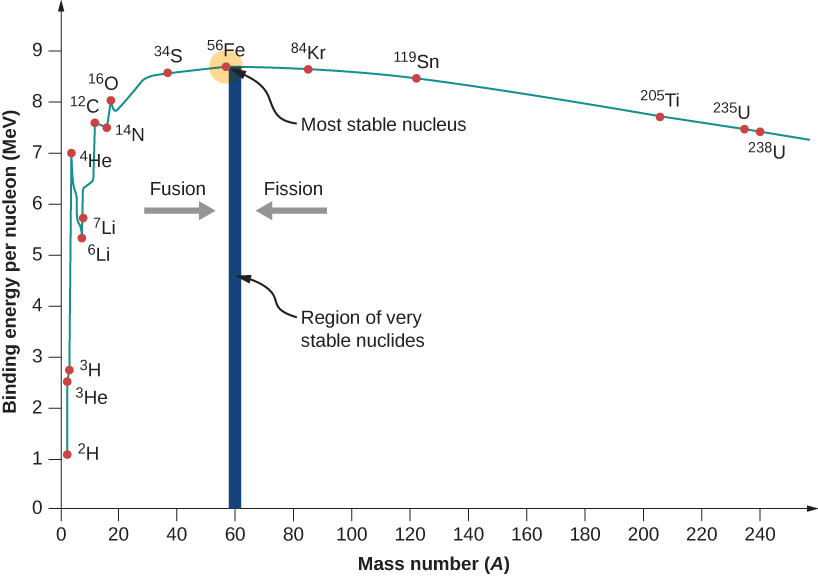
- For medium-sized nuclei (30 < A < 170), the binding energy per nucleon is almost constant (~8.75 MeV for A = 56).
- Binding energy per nucleon is lower for both very light nuclei (A < 30) and very heavy nuclei (A > 170).
Important Conclusions:
- The nuclear force is strong and attractive, providing a binding energy of a few MeV per nucleon.
- The binding energy per nucleon is constant for nuclei of medium mass numbers, which reflects the short-range nature of the nuclear force.
- Saturation Property of the Nuclear Force:
- Each nucleon only interacts with a limited number of neighboring nucleons within the range of the nuclear force.
- Fission of Heavy Nuclei:
- Heavy nuclei (like A = 240) have lower binding energy per nucleon compared to A = 120.
- If a nucleus with A = 240 breaks into two A = 120 nuclei, energy is released (because the binding energy per nucleon increases).
- This is the basis for nuclear fission and energy release in nuclear reactors.
- Fusion of Light Nuclei:
- In nuclear fusion, two very light nuclei (e.g., A ≤ 10) combine to form a heavier nucleus.
- The binding energy per nucleon of the fused nucleus is higher, meaning energy is released.
- This is the energy source of the sun.
Nuclear Force
- The nuclear force is the force that binds protons and neutrons together within the nucleus of an atom.
- It is one of the four fundamental forces of nature, along with gravitational, electromagnetic, and weak forces.
- The nuclear force operates at extremely short distances, typically less than 1 femtometer (10-15 meters), and is responsible for holding the nucleus together despite the repulsive electrostatic force between positively charged protons.
- Nuclear force is responsible for the binding energy per nucleon (~8 MeV), which is much larger than the binding energy in atoms.
Key Characteristics
- Short-Range: Significant only at distances of about 1-2 femtometers.
- Attractive and Repulsive:
- Repulsive at very short distances (around 0.5 femtometers).
- Attractive at slightly larger distances (1-2 femtometers), overcoming the repulsive electrostatic force between protons.
- Independent of Charge: Works equally between protons and neutrons, unlike electromagnetic force.
- Exchange Force: Mediated by particles like pions (π-mesons)
Radioactivity
Why Do Nuclei Decay?
- Nuclei decay because they are unstable. This instability arises from the balance between two fundamental forces.
- When the nucleus becomes too heavy (i.e., it has too many protons), the strong nuclear force is no longer sufficient to counteract the electrostatic repulsion between protons. This imbalance leads to nuclear instability, which can result in decay.
Radioactivity
- Radioactivity is the spontaneous emission of particles or radiation from the nucleus of an unstable atom to achieve a more stable configuration.
Decay Modes:
- Alpha Decay: Emission of an alpha particle (2 protons, 2 neutrons).
- Beta Decay: Conversion of a neutron to a proton (β⁻) or a proton to a neutron (β⁺).
- Gamma Decay: Emission of high-energy photons without changing the nucleus composition.
- Spontaneous Fission: Splitting of a heavy nucleus into smaller nuclei, releasing energy
The Law of Radioactive Decay:
Radioactive decay follows an exponential decay law, where the number of nuclei disintegrating per unit time is proportional to the current number of undecayed nuclei in the sample.
The differential form of the decay law is:
ΔN/Δt = −λN
Where:
- N = Number of undecayed nuclei at time t
- λ = Decay constant (specific to each radionuclide)
The number of remaining nuclei over time is given by:
N(t)=N0e−λt
Where N0 is the initial number of nuclei.
Radioactive Activity:
- Activity (R): The number of disintegrations per unit time, or the rate of decay, is termed as activity.
- Its SI unit is becquerel (Bq), where:
1 Bq=1 decay per second
A larger unit, curie (Ci), is also used:
1 Ci=3.7×1010 Bq
- A (or R)=λN
Where:
- A: Activity (measured in becquerels or curies)
- N: Number of undecayed nuclei
Half-life and Mean Life:
- Half-life (T1/2): The time it takes for half of the initial nuclei in a sample to decay. It is related to the decay constant (λ) by:
T1/2 = ln(2) / λ = 0.693 / λ
- Mean Life (τ): The average lifetime of a nucleus before it decays. It is the inverse of the decay constant:
τ = 1/λ
Radioactive Decay Types
Alpha Decay (α-decay):
- Example: Decay of Uranium-238 (²³⁸U) to Thorium-234 (²³⁴Th) with emission of a helium nucleus (⁴He).
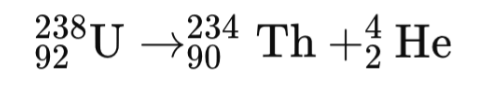
- General form:
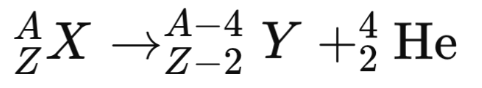
- Mass number decreases by 4 and atomic number decreases by 2.
- The Q-value of the reaction is the difference in mass energy between the initial and final products:
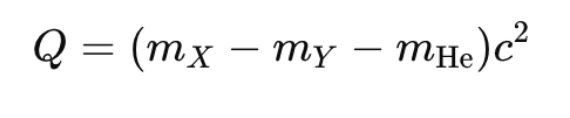
Beta Decay (β-decay):
- β⁻ decay: A neutron converts into a proton while emitting an electron (e⁻) and an antineutrino (ν).
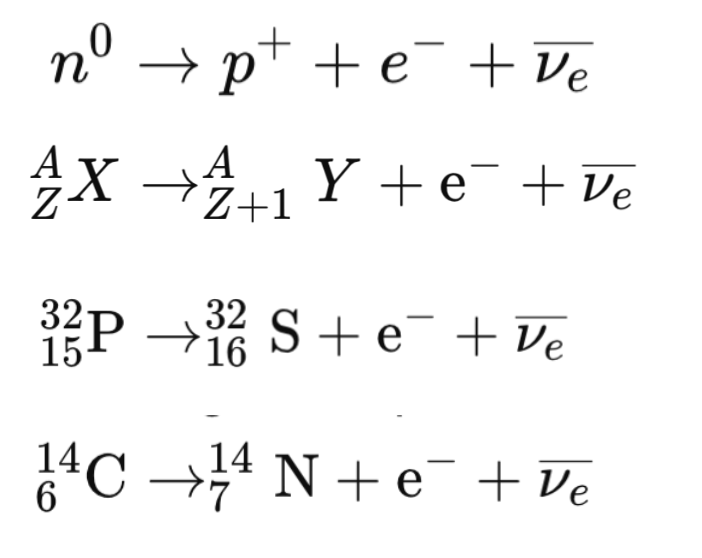
- β⁺ decay: A proton converts into a neutron while emitting a positron (e⁺) and a neutrino (ν).
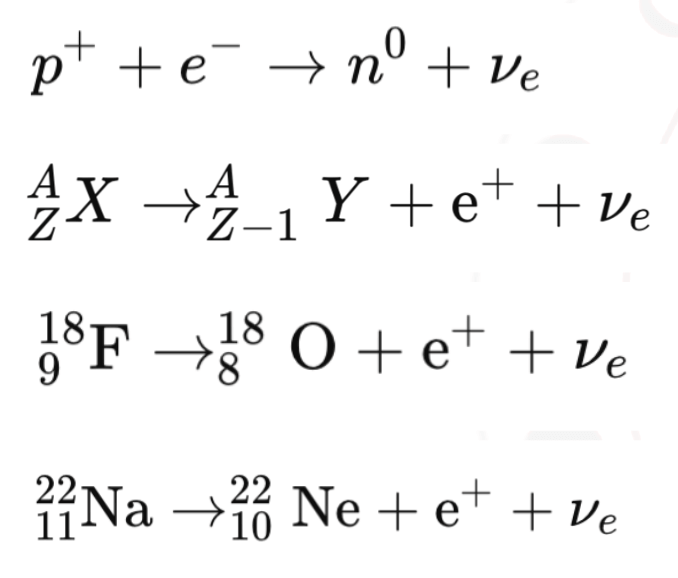
Gamma Decay (γ-decay):
- Nuclei have discrete energy levels (similar to atomic levels).
- Gamma decay occurs when a nucleus in an excited state decays to its ground state by emitting a photon(gamma ray) with energy equal to the difference between the two energy levels.
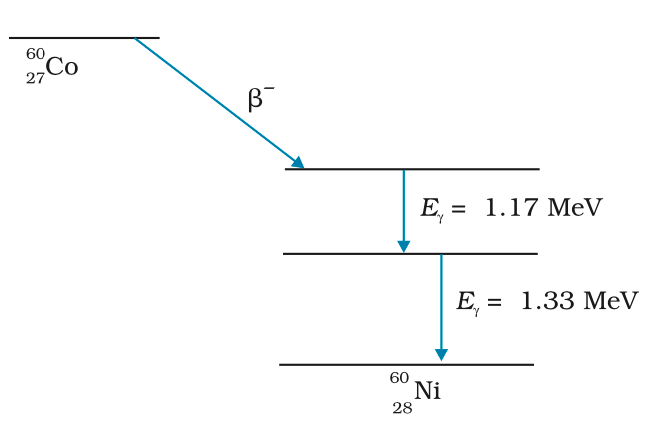
- Example: After β-decay, the daughter nucleus may be in an excited state, and it may release energy through gamma radiation to return to the ground state.
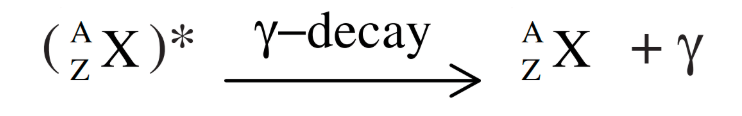
Nuclear Energy
Nuclear energy is the energy stored in the nucleus of an atom, released during nuclear reactions like fission and fusion.
- Lighter Nuclei (A < 30) & Heavier Nuclei (A > 170): These nuclei have binding energy per nucleon less than 8.0 MeV, meaning less stability.
- Fission (heavy nuclei split into lighter ones) and Fusion (light nuclei fuse into heavier ones) result in a net energy release due to an increase in binding energy per nucleon.
Comparison with Chemical Energy:
- Nuclear reactions release a million times more energy (order of MeV ) than chemical reactions (order of eV) for the same quantity of matter.
- Example: Fission of 1 kg of uranium releases 1014 J, while burning 1 kg of coal releases only 107 J.
Key Types of Nuclear Reactions
- Fusion and Fission
Nuclear Fission
- Definition: Fission is the process where a heavy atomic nucleus, such as uranium-235 or plutonium-239, splits into two smaller nuclei, releasing energy, neutrons, and radiation.
- The energy released comes from the binding energy of the atoms involved, which is released when the nucleus splits.
Discovery:
- Nuclear fission was discovered in 1938 by German scientists Otto Hahn and Fritz Strassmann.
- The theoretical explanation was provided by Lise Meitner and Otto Frisch, who correctly interpreted the process as the splitting of an atom.
Process of Nuclear Fission
- Neutron Absorption:
- A neutron collides with a heavy nucleus (e.g., uranium-235).
- Nucleus Splitting:
- The nucleus splits into 2 smaller nuclei (fission fragments), releasing energy.
- Neutron Release:
- 2-3 additional neutrons are released → can trigger more fission reactions → chain reaction.
- Energy Release:
- Energy is released in the form of kinetic energy of the fission fragments, gamma radiation, and the release of additional neutrons. The energy released is enormous and is a result of the conversion of mass to energy, as described by Einstein’s equation E=Δmc2
- The energy released (the Q value ) in the fission reaction of nuclei like uranium is of the order of 200 MeV per fissioning nucleus.
Example: Uranium-235 absorbs a neutron → Becomes unstable → Splits into smaller nuclei + energy.

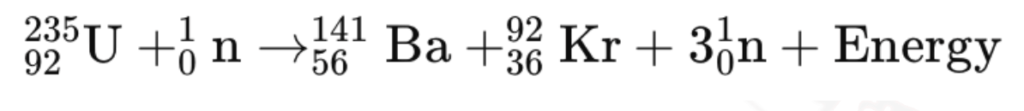
Mass Defect and Binding Energy:
- Mass Defect: The mass of the fission fragments and released neutrons is less than the mass of the original nucleus. This difference in mass is converted into energy, as per Einstein’s mass-energy equivalence: E=mc2
- Fission increases the total binding energy of the resulting nuclei (the products), compared to the original heavy nucleus — and that increase in binding energy is the energy released.
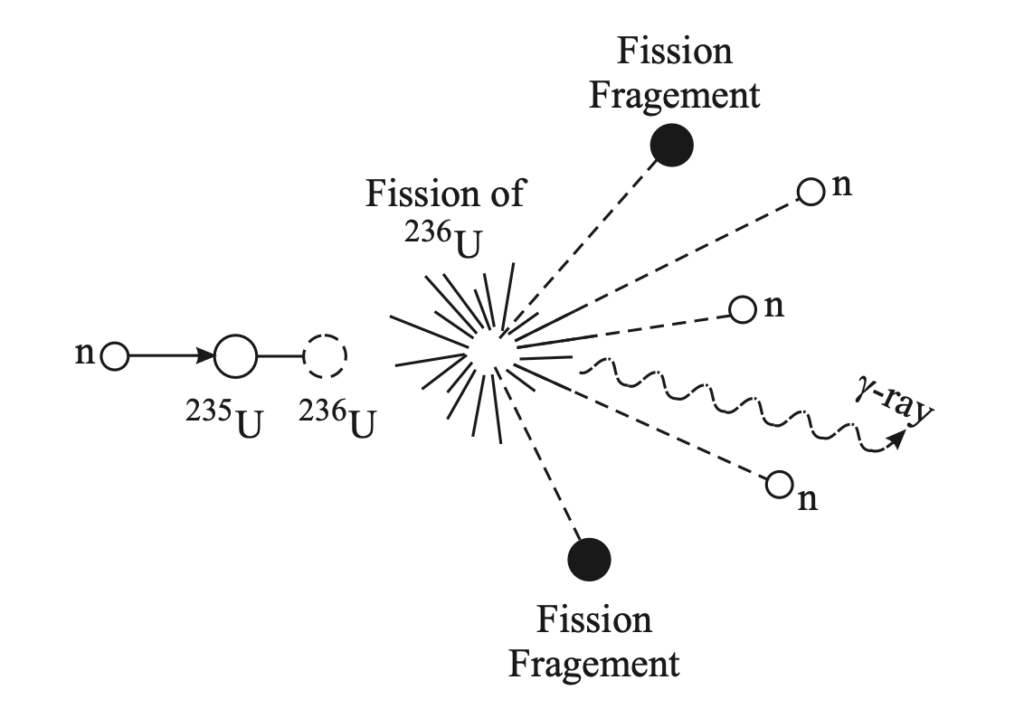
Chain Reaction in Nuclear Fission
- Self-Sustaining Reaction:
- A chain reaction occurs when the neutrons released from one fission event trigger subsequent fission events in nearby nuclei. This is the foundation of the energy generation process in nuclear reactors.
- Controlled Chain Reaction (in a nuclear reactor) – The reaction is sustained at a steady rate, providing continuous energy.
- Uncontrolled Chain Reaction (in an atomic bomb) – The reaction escalates rapidly, releasing an enormous amount of energy in a very short time.
- In a nuclear reactor, a controlled chain reaction is maintained. Example:
- A uranium-235 nucleus absorbs a neutron and splits.
- The splitting releases 2-3 neutrons, which go on to split more uranium nuclei.
- This cycle continues, causing a large amount of energy to be released.
- Critical Mass:
- For a chain reaction to continue, there must be a sufficient amount of fissionable material. This critical amount of material is called critical mass.
- If the amount of material is too small, the chain reaction will not sustain itself. If the material is too large, the reaction may escalate uncontrollably (as in a nuclear explosion).
- Control of the Chain Reaction:
- In nuclear reactors, the chain reaction is carefully controlled using control rods (made from neutron-absorbing materials like boron or cadmium), which regulate the number of free neutrons and prevent the reaction from going too fast.
Applications of Nuclear Fission
- Nuclear Power:
- Power Plants: Heat from fission generates steam → Drives turbines → Electricity.
- Nuclear Weapons:
- Atomic Bombs: The bomb uses the rapid, uncontrolled chain reaction of uranium-235 or plutonium-239 to release an enormous amount of energy in the form of an explosion.
- Medical Isotope Production:
- Fission reactors produce isotopes for medical imaging (e.g., iodine-131).
- Space Exploration:
- RTGs (Radioisotope Thermoelectric Generators) for powering spacecraft (e.g., Voyager).
Nuclear Reactor
What is a Nuclear Reactor?
- A nuclear reactor is a device used to sustain and control a chain reaction of nuclear fission to produce energy, typically in the form of heat.
- The heat generated in a nuclear reactor is used to produce steam, which drives turbines connected to electricity generators.
A Controlled Fission Reaction
- When a nucleus like Uranium-235 undergoes fission, it releases extra neutrons (typically 2-3 per fission), which can initiate further fission reactions. This process, if uncontrolled, can lead to explosive energy release, as seen in a nuclear bomb.
However, in a nuclear reactor, the chain reaction must be controlled:
- Neutron Moderators: To sustain the chain reaction, neutrons must be slowed down to increase the likelihood of further fission. This is done by using moderators such as water, heavy water (D₂O), or graphite, which slow down fast neutrons.
- Control Rods: To control the reaction, control rods made of neutron-absorbing materials like cadmium are inserted into the reactor to absorb excess neutrons and regulate the rate of the reaction.
- Critical, Supercritical, and Subcritical:
- If the multiplication factor K (the ratio of neutrons from one generation to the next) is exactly 1, the reactor is in a critical state, providing a steady power output.
- If K>1, the reaction becomes supercritical, leading to exponentially increasing energy output, which can be dangerous.
- If K<1, the reaction will slow down and eventually stop.
The Chernobyl disaster in 1986 was a tragic reminder of the potential dangers of uncontrolled chain reactions in a nuclear reactor.
Plutonium Production:
- When 238U captures a neutron, it forms plutonium through the following reactions:
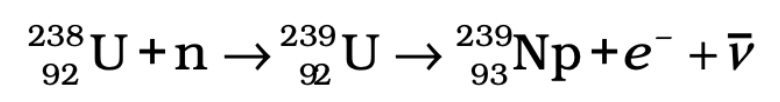
- Plutonium can undergo fission with slow neutrons, providing another fuel source.
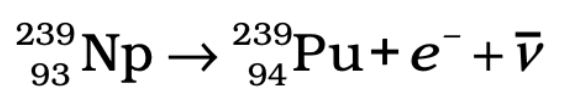
U-238 → U-239 → Neptunium-239 →Plutonium-239
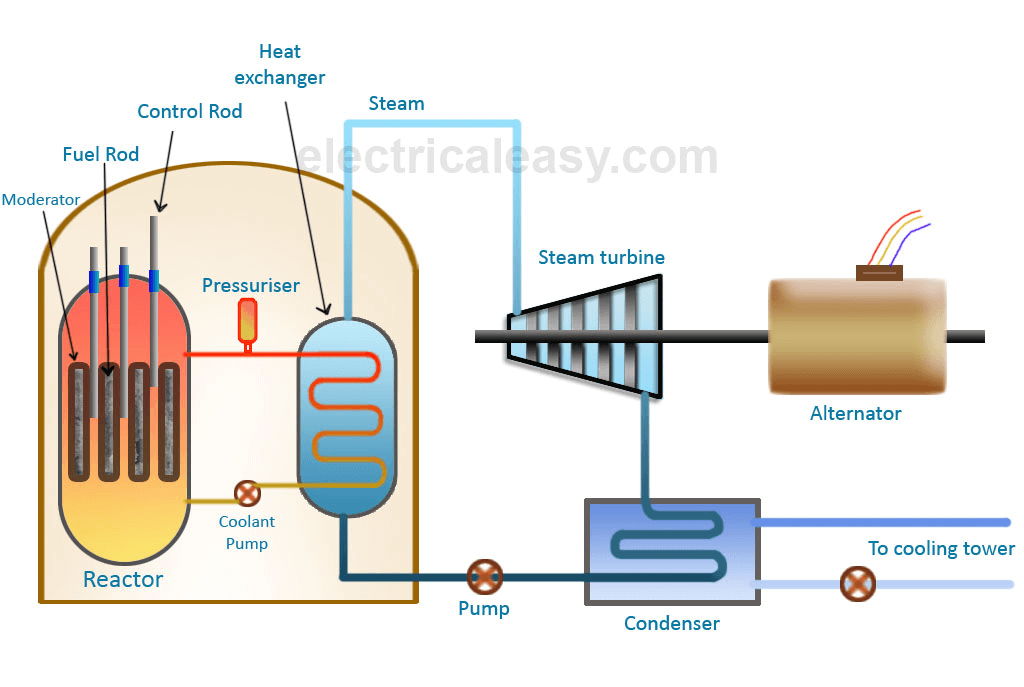
Basic Components of a Nuclear Reactor
- Fuel Rods (Fissile Material)
- Contain uranium-235 or plutonium-239, which undergo fission when struck by neutrons.
- Arranged in the reactor core to sustain the chain reaction.
- Moderator
- Slows down fast-moving neutrons to increase the chances of further fission.
- Common moderators: Heavy water (D₂O), Graphite, or Ordinary water (H₂O).
- Control Rods
- Regulate the reaction by absorbing excess neutrons, preventing an uncontrolled reaction.
- Made of materials like boron, cadmium, or hafnium.
- Coolant
- Transfers heat from the reactor core to a steam generator.
- Common coolants: Water, liquid sodium, or gas (CO₂, helium).
- Pressure Vessel
- Encases the reactor core, providing structural integrity and safety.
- Steam Generator & Turbine System
- Heat from fission boils water to produce steam, which drives turbines to generate electricity.
- Containment Structure
- A reinforced concrete shield that protects against radiation leaks.
How Does a Nuclear Reactor Work?
- Fuel Rods → Contain fissile material (Uranium-235, Plutonium-239).
- Neutron Strikes Fuel → Fission reaction releases energy + neutrons.
- Moderator (Water/Graphite) → Slows down neutrons to sustain chain reaction.
- Control Rods (Boron/Cadmium) → Absorb excess neutrons to regulate reaction.
- Coolant (Water/Liquid Metal) → Transfers heat from reactor core to steam generator.
- Steam → Turbine → Generator → Electricity production.
Types of Nuclear Reactors
Pressurized Water Reactor (PWR):
- Description: The most common type of reactor worldwide. In a PWR, the reactor core is submerged in water under high pressure, which prevents it from boiling even at high temperatures.
- Key Features:
- Two separate water circuits: One circulates within the reactor core (primary circuit), and the other is used to generate steam (secondary circuit).
- The primary coolant is pressurized to maintain its liquid state, even at high temperatures.
- The secondary circuit is separate, preventing contamination of the steam used to turn the turbine.
Boiling Water Reactor (BWR):
- Description: In a BWR, water is allowed to boil directly inside the reactor vessel, producing steam that drives the turbine.
- Key Features:
- Single-loop system: The same water that cools the reactor core is converted to steam and used to drive the turbine.
- Simpler design compared to PWRs, but with more direct exposure to radioactive materials in the steam.
Fast Breeder Reactor (FBR):
- Description: FBRs use fast neutrons and a liquid metal coolant (typically sodium). These reactors are designed to produce more fissile material than they consume, by converting non-fissile uranium-238 into plutonium-239.
- Key Features:
- Can use depleted uranium as fuel.
- Breeds fuel by converting uranium-238 into plutonium-239, effectively “breeding” more fuel for future use.
Heavy Water Reactor (HWR):
- Description: Uses deuterium oxide (heavy water, D20) as the moderator instead of light water (H2O).
- Key Features:
- Heavy water is more efficient in slowing down neutrons, allowing the use of natural uranium as fuel.
- Commonly used in countries like Canada and India.
Gas-Cooled Reactor
- Uses helium or CO₂ as a coolant.
- Operates at higher efficiency with less water usage.
Reactor Safety & Control
- Control Rods → Adjust reaction rate (insert = slow down, remove = speed up).
- Cooling System → Prevents overheating and meltdown.
- SCRAM (Emergency Shutdown) → Instantly stops the reaction if needed.
- Containment Structure → Protects against radiation leaks.
Applications of Nuclear Fission
1. Power Generation (Nuclear Energy)
- How? → Controlled fission in nuclear reactors produces heat → Steam → Turbine → Electricity.
- Example: Kudankulam, Tarapur, Rawatbhata (India’s nuclear power plants).
- Why?
- High energy output (1 kg uranium = 3 million kg coal).
- Low carbon emissions.
- Reliable energy source (continuous supply).
- Challenges?
- Radioactive waste disposal.
- High setup cost.
- Safety concerns (Chernobyl, Fukushima).
2. Medical Applications (Nuclear Medicine)
- How? → Fission byproducts (radioisotopes) are used in medicine.
- Examples:
- Cancer Treatment (Radiotherapy) → Cobalt-60, Cesium-137.
- Medical Imaging → Technetium-99m (detects tumors, heart issues).
- Sterilization → Radiation used to sterilize medical equipment.
- Why?
- Precise treatment for tumors.
- Non-invasive diagnosis.
- Ensures sterile medical tools.
- Precise treatment for tumors.
3. Industrial & Agricultural Applications
- How? → Radiation from fission helps in testing, preservation, and quality control.
- Examples:
- Non-Destructive Testing (NDT) → X-ray imaging of pipelines, bridges.
- Food Irradiation → Kills bacteria, increases shelf life.
- Mutation Breeding → Creates disease-resistant crops.
4. Space Exploration (Nuclear Propulsion & Power)
- How? → Fission reactors provide energy in space missions.
- Examples:
- RTGs (Radioisotope Thermoelectric Generators) → Used in Mars Rovers (Curiosity, Perseverance).
- Nuclear Propulsion → Faster travel in deep space (NASA’s NTP research).
5. Military Use (Nuclear Weapons)
- How? → Uncontrolled fission reactions release massive energy (atomic bombs).
- Examples: Hiroshima (Uranium-235) & Nagasaki (Plutonium-239).
- Why Controversial?
- High destruction potential.
- Long-term environmental & health impacts.
- Leads to global arms race.
Challenges and Risks
- ⚠️ Radioactive Waste – Requires long-term storage and disposal.
- ⚠️ Risk of Accidents – Chernobyl (1986) & Fukushima (2011) highlight safety concerns.
- ⚠️ High Initial Cost – Expensive to build and maintain.
Nuclear Fusion
Nuclear fusion is a process in which two light atomic nuclei combine to form a heavier nucleus, releasing an enormous amount of energy. Fusion is the process that powers the Sun and other stars by converting hydrogen into helium.
- Fusion typically occurs between isotopes of hydrogen:
- Deuterium (²H) – found in seawater.
- Tritium (³H) – produced artificially in nuclear reactions.
Example Reactions:
1. Proton-Proton Fusion (Sun’s energy source):
Two protons combine to form deuterium, releasing energy in the form of a positron and neutrino).
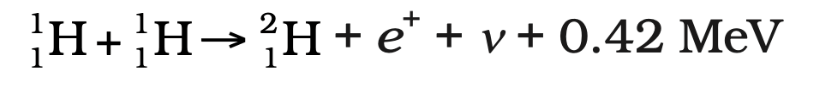
Energy Released in Nuclear Fusion
- Fusion reactions release 4-5 times more energy than nuclear fission.
- Example: The fusion of 1 kg of deuterium and tritium releases 10 million times more energy than burning 1 kg of coal.
Takes billions of years to complete, making it unsuitable for Earth-based energy production.
2. Deuterium-Deuterium Fusion:
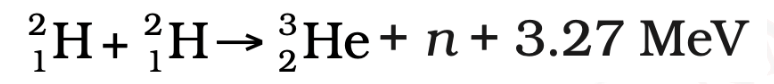
3. Deuterium-Tritium Fusion:
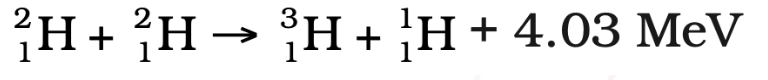
Energy Released in Nuclear Fusion
- Fusion reactions release 4-5 times more energy than nuclear fission.
- Example: The fusion of 1 kg of deuterium and tritium releases 10 million times more energy than burning 1 kg of coal.
Comparison of Energy Output (Per Reaction):
| Reaction Type | Energy Released (MeV) |
| Chemical (e.g., burning fuel) | 1 eV |
| Nuclear Fission | 200 MeV |
| Nuclear Fusion | 17.6 MeV |
Discovery & Research:
- The concept of nuclear fusion was first understood in the 1920s when Arthur Eddington proposed that the Sun’s energy comes from the fusion of hydrogen into helium.
- Fusion research gained momentum in the 1950s with the development of hydrogen bombs and later peaceful energy projects.
How Does Nuclear Fusion Work?
- Collision of Light Nuclei →Deuterium and tritium nuclei come close.
- Overcoming Electrostatic Repulsion → Positive charges cause repulsion, but high speeds (100 million°C) help overcome it.
- Formation of a New Nucleus → The nuclei merge to form a heavier nucleus (e.g., helium).
- Release of Energy → The mass of the fused nucleus is less than the sum of the original masses.
- The lost mass is converted into energy:
E=mc2
- The lost mass is converted into energy:
Conditions Required for Fusion
- Extremely High Temperature (10-100 million °C): Since nuclei are positively charged, they experience Coulomb repulsion. ➔ High temperature is required to provide enough kinetic energy to overcome this barrier. ➔ Fusion temperature is around 3×109 K.
- The Sun achieves this through gravitational pressure. On Earth, we use high-energy lasers or magnetic confinement
- High Pressure → Bring nuclei close for fusion.
- In stars, gravity compresses the core; in experimental reactors, powerful magnetic fields or lasers are used.
- Plasma State → Ionized gas where nuclei can collide freely.
- At high temperatures, atoms lose their electrons, forming a plasma (a state of matter where nuclei and electrons move freely).
- Plasma allows controlled fusion reactions in reactors like Tokamaks and Stellarators.
- Confinement Time → The reacting nuclei must be held together long enough for fusion to occur.
Where Does Fusion Occur?
✅ Naturally in Stars (Sun, other stars).
✅ Artificially in Labs (Tokamak, Stellarator).
Thermonuclear fusion
When fusion is achieved by raising the temperature of the system so that particles have enough kinetic energy to overcome the Coulomb repulsive behaviour, it is called thermonuclear fusion.
- Thermonuclear fusion is the source of energy output in the interior of stars.
Controlled Thermonuclear Fusion:
- In stars, natural fusion occurs at high temperatures (~10⁸ K), forming plasma.
- Controlled fusion aims to replicate this process to generate steady power.
- The challenge is confining plasma at such high temperatures, as no container can withstand it.
Fusion in the Sun:
The core of the Sun reaches temperatures of about 1.5×10⁷ K, which is sufficient for thermonuclear fusion. The proton-proton cycle in the Sun involves several steps where hydrogen is fused into helium, releasing 26.7 MeV of energy for every 4 hydrogen nuclei that fuse.
Proton-Proton Cycle in the sun:
Step 1: Proton-Proton Fusion (Hydrogen to Deuterium)
- Two protons collide at high temperatures (~15 million°C) and fuse together.
- One of the protons undergoes beta-plus decay and transforms into a neutron, forming deuterium (²H), a hydrogen isotope.
- This reaction also releases a positron (e⁺) and a neutrino (νₑ).
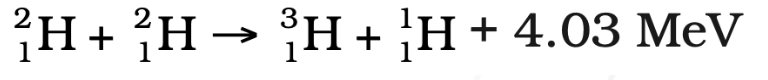
Positron-Electron Annihilation
- During Step 1, when a proton decays into a positron (e⁺), the positron soon encounters an electron (e⁻) in the Sun’s core.
- This leads to positron-electron annihilation, producing two gamma photons and releasing 1.02 MeV of energy.
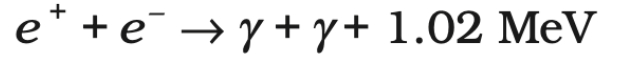
Step 2: Deuterium and Proton Fusion (Deuterium to Helium-3)
- The deuterium nucleus (²H) formed in the previous step fuses with another proton (p), forming helium-3 (³He).
- This reaction releases a gamma-ray photon (γ), which carries energy.
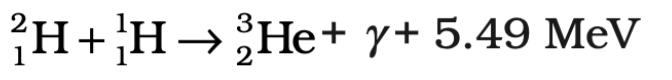
Step 3: Helium-3 Fusion (Helium-3 to Helium-4)
- Two helium-3 nuclei (³He) then collide and fuse, forming helium-4 (⁴He).
- This process releases two protons as byproducts.

Overall Fusion Equation in the Sun:
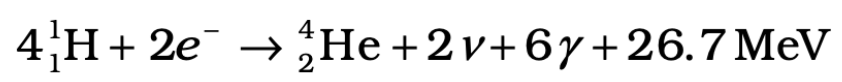
- Four hydrogen atoms combine to form helium and release 26.7 MeV of energy.
Energy Release: How Fusion Powers the Sun
- The energy released from these fusion reactions comes from the mass defect: the mass of the helium nucleus is slightly less than the total mass of the four protons that fused.
- This missing mass is converted into energy (according to Einstein’s equation E = mc²).
- The gamma rays produced during the reactions travel outward from the Sun’s core, eventually reaching the surface, where they are emitted as visible light and heat.
Energy Production in Stars:
- Fusion in stars like the sun is a multi-step process that burns hydrogen into helium.
- As the hydrogen in the core gets depleted, the core temperature increases, and helium fusion begins, producing heavier elements such as carbon.
Future of the Sun:
- The sun’s fuel will last for another 5 billion years.
- After the hydrogen runs out, the sun will expand into a red giant and eventually collapse.
Nuclear Holocaust
Energy from Fission:
- In a single uranium fission, ~200 MeV of energy is released.
- If 50 kg of Uranium-235 undergoes fission, the energy released is ~4 × 10¹⁵ J, equivalent to 20,000 tons of TNT.
- This energy is enough for a super-explosion, called an atomic explosion.
- On August 6, 1945, the U.S. dropped an atomic bomb on Hiroshima, with the same explosive
- power (20,000 tons of TNT). It instantly devastated 10 sq km, killing 66,000 and injuring 69,000 people.
Hydrogen Bomb (Fusion Bomb):
- Hydrogen bombs use isotopes of hydrogen (deuterium and tritium) in a fusion process.
- Super-explosions of up to 10 megatons of TNT equivalent were tested in 1954.
- A nuclear holocaust, triggered by such bombs, could destroy all life on Earth and cause a nuclear winter, where radioactive fallout blocks sunlight and makes the planet uninhabitable.
Why Is Fusion the Future of Energy?
1. Unlimited Fuel Source
- Deuterium is abundant in seawater (1 liter of seawater has enough deuterium for 300 years of electricity per person).
- Tritium can be bred from lithium, which is widely available.
2. No Long-Lived Radioactive Waste
- Unlike fission, fusion produces minimal nuclear waste and no long-lived radioactive byproducts.
3. Extremely High Energy Output
- 1 gram of fusion fuel releases energy equal to burning 8,000 liters of oil!
- Much more efficient than fossil fuels and fission.
4. No Risk of Meltdown
- Unlike fission reactors (which can overheat and cause disasters), fusion reactors automatically shut down if conditions are not met.
5. No Greenhouse Gas Emissions
- 100% clean energy with zero carbon emissions.
Challenges in Achieving Practical Fusion Energy
1. Sustaining High Temperatures & Pressures
- Plasma must be maintained at millions of degrees Celsius without losing energy.
2. Plasma Confinement Issues
- Magnetic confinement (Tokamak) and inertial confinement (lasers) are being developed, but both require extreme precision.
3. Material Limitations
- Reactor walls must withstand neutron bombardment and extreme heat.
4. Tritium Availability
- Tritium is scarce and radioactive. Breeding it from lithium is being explored.
5. Cost & Complexity
- Current projects (like ITER) cost billions of dollars with no commercial fusion plant expected before 2050.
Artificial Fusion and Research
Since fusion has immense potential as a clean energy source, scientists are working on developing controlled nuclear fusion.
Two main approaches:
- Magnetic Confinement (Tokamak Reactors):
- Uses powerful magnetic fields to contain the hot plasma needed for fusion.
- Example: ITER (International Thermonuclear Experimental Reactor). → Aims to produce 10 times more energy than input by 2035.
- Inertial Confinement (Laser-Based Fusion):
- Uses high-powered lasers to compress and heat fuel to fusion conditions.
- Example: National Ignition Facility (NIF), USA → Achieved fusion ignition in 2022 (first-ever net energy gain from fusion).
- SPARC Reactor (MIT & Commonwealth Fusion Systems, USA)
- Private sector-led compact Tokamak design.
- Aims for a commercial fusion plant by 2035.
- India’s Fusion Research
- Participates in ITER.
- Developing SST-1 (Steady State Superconducting Tokamak) at IPR (Institute for Plasma Research).
Comparison Between Nuclear Fission and Nuclear Fusion
| Aspect | Nuclear Fission | Nuclear Fusion |
| Process | Splitting of heavy atomic nuclei (e.g., uranium, plutonium). | Joining of light atomic nuclei (e.g., deuterium, tritium). |
| By-products | Radioactive waste (e.g., spent fuel, nuclear waste), hazardous for thousands of years. | Primarily helium (harmless), some short-lived isotopes. |
| Fuel | Uranium-235 (natural and enriched), Plutonium-239. | Deuterium (from seawater), Tritium (breeding from Lithium). |
| Energy Input for Reaction | Minimal energy required (neutron bombardment). | Requires extremely high temperature (~100 million °C) and pressure. |
| Energy per Reaction | 200 MeV per fission of Uranium-235. | 17.6 MeV per fusion of Deuterium and Tritium. |
| Energy per kg of Fuel | 80,000 MWh per ton of Uranium-235. | >10,000,000 MWh per gram of fuel. |
| Current Status | Used in 440 reactors globally, supplying 15% of global electricity, including 72% of France’s energy. | Experimental; no commercial reactors yet, prototypes like ITER.ITER aims for 500 MW fusion power by 2035 |
| Environmental Impact | Low emissions, 0.02 kg CO2/kWh, but long-lived radioactive waste. | Zero emissions during operation, minimal radioactive waste. |
| Fuel Availability | Uranium reserves may last 80-100 years at current consumption rates. | Deuterium is abundant, and Tritium can be bred, making fusion fuel virtually limitless. |
| Safety Risk | Accidents like Chernobyl (1986) and Fukushima (2011), nuclear proliferation. | No risk of runaway reactions, inherently safer. |
| Energy Efficiency | Current reactors are about 33-37% efficient. | Potentially 100% efficient. |
| First Controlled Reaction | 1942 (Chicago Pile-1, USA). | Expected 2025-2035 (ITER aims for continuous fusion). |
| Notable Reactors | Chernobyl, Fukushima, San Onofre, Hinkley Point C. | ITER (France), National Ignition Facility (USA). |
| Radioactive Waste | Long-lived by-products, global stockpile exceeds 250,000 tons. | Minimal waste, short-lived isotopes produced. |
| Global Energy Generation | 6,000 TWh/year globally from fission reactors. | Fusion could provide 10,000 times more energy than current global demand. |
| Cost of Fuel | Expensive, uranium mining, and enrichment costs. | Fuel is essentially free or low-cost; seawater for deuterium, lithium for tritium. |
Atomic Energy Programme
In the 1950s, Dr. Homi Bhabha, the father of the Indian nuclear program, conceptualized India’s three-stage nuclear program.
Objective Behind India’s Nuclear Power Program
- India has one of the biggest shares of worldwide Thorium reserves as opposed to a small share of worldwide Uranium reserves. But, Thorium is not a fissile material. But, it can breed another fissile material, U-233. U-233 is the most used nuclear fuel.
- Breeding of U-233 and achieving a Thorium fuel cycle is not a one-step process, that is why India needs a three-stage program. So, the objective is to achieve the Thorium fuel cycle and be self-sufficient in meeting energy demands.
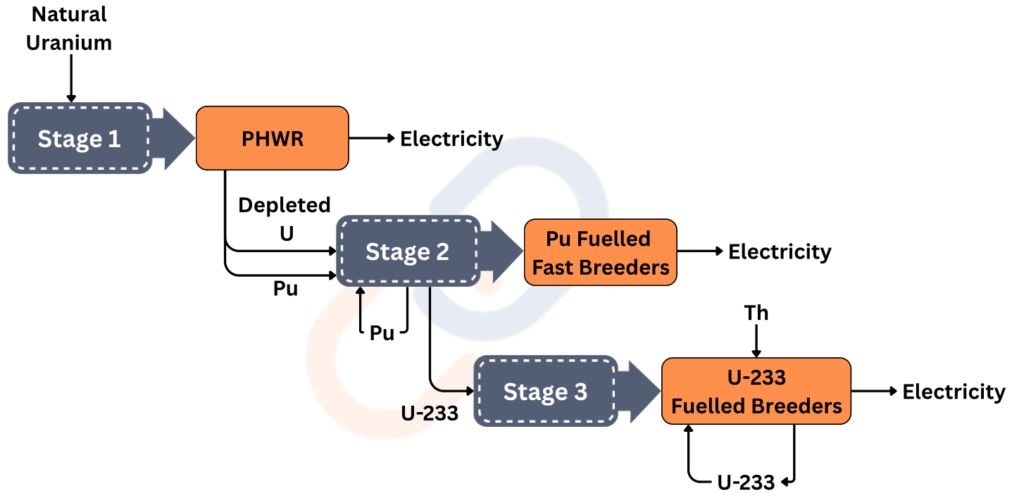
Stage I: Pressurized Heavy Water Reactors (PHWRs)
- Fuel: Natural uranium (U-238)
- Moderator & Coolant: Heavy water (D₂O)
- Output: Electricity and plutonium-239 (Pu-239)
- Significance: PHWRs efficiently utilize natural uranium without the need for enrichment, making them cost-effective and suitable for India’s resource profile.
- Current Status: India operates 22 nuclear reactors, including 18 PHWRs and 4 Light Water Reactors (LWRs), with a combined capacity of approximately 8,180 MW as of January 2025
Stage II: Fast Breeder Reactors (FBRs)
- Fuel: Plutonium-239 (from Stage I) mixed with uranium-238
- Coolant: Liquid sodium
- Output: Electricity and additional plutonium-239
- Significance: FBRs are designed to generate more fissile material than they consume, effectively “breeding” fuel and enhancing sustainability.
- Current Status: The Prototype Fast Breeder Reactor (PFBR) at Kalpakkam, Tamil Nadu, with a capacity of 500 MW, began core loading in March 2024 and is expected to be operational by the end of 2025 . Plans are underway to construct additional FBRs, including the FBR-600 series, to further this stage.
Stage III: Thorium-Based Reactors
- Fuel: Thorium-232 converted to uranium-233 (U-233)
- Reactor Type: Advanced Heavy Water Reactor (AHWR)
- Significance: This stage aims to establish a self-sustaining thorium fuel cycle, leveraging India’s vast thorium reserves for long-term energy security.
- Current Status: The AHWR is in the design and development phase, with a prototype planned to demonstrate thorium utilization technologies . Full-scale deployment is anticipated post-2030, following successful demonstration and validation.
Future Outlook
India has set an ambitious target to achieve 100 GW of nuclear power capacity by 2047, up from the current 8.2 GW. To facilitate this expansion, the government is considering reforms to allow greater private and foreign investment in the nuclear sector, including amending liability laws and permitting up to 49% foreign equity in nuclear projects .
FAQ (Previous year questions)
Aspect
Nuclear Fission
Nuclear Fusion
Process
Splitting of heavy atomic nuclei (e.g., uranium, plutonium).
Joining of light atomic nuclei (e.g., deuterium, tritium)
Fuel
Uranium-235 (natural and enriched), Plutonium-239.
Deuterium (from seawater), Tritium (breeding from Lithium).
Energy per Reaction
200 MeV per fission of Uranium-235.
17.6 MeV per fusion of Deuterium and Tritium.
By-products
Radioactive waste (e.g., spent fuel, nuclear waste), hazardous for thousands of years.
Primarily helium (harmless), some short-lived isotopes.
β⁻ Decay (Beta Minus Decay):
A neutron inside the nucleus converts into a proton.It emits a beta particle (electron, e⁻) and an antineutrino (ν̅).
Process:
β⁺ Decay (Beta Plus Decay):
A proton inside the nucleus converts into a neutron.It emits a positron (e⁺) and a neutrino (ν).
Process:
Nuclear fission and Fusion /Nuclear fission and Fusion /Nuclear fission and Fusion /Nuclear fission and Fusion /Nuclear fission and Fusion /Nuclear fission and Fusion /Nuclear fission and Fusion /Nuclear fission and Fusion/Nuclear fission and Fusion
